COVID-19 informazioni
Sei alla ricerca di informazioni sull’utilizzo delle nostre soluzioni durante la pandemia COVID-19? Troverai di seguito alcuni aggiornamenti.
Attenzione: i contenuti di questa sezione sono in lingua inglese
ResMed si è impegnata a consegnare forniture per l’assistenza respiratoria ai sistemi sanitari e ai paesi di tutto il mondo, proprio come fa da decenni.
Oggi, mentre il coronavirus (COVID-19) continua a diffondersi in tutto il pianeta, siamo vicini a tutti i professionisti sanitari che curano i pazienti con bisogni assistenziali complessi.
Il nostro impegno di lunga data nel migliorare la vita delle persone con problemi respiratori è risultato utile in questi tempi difficili. Facciamo del nostro meglio per comunicare con la nostra rete di clienti per supportarli, coinvolgerli e formarli su come le soluzioni terapeutiche essenziali possono essere fornite il più rapidamente possibile.
Le nostre soluzioni terapeutiche di ventilazione invasiva e non invasiva, terapia CPAP, ossigenoterapia, maschere e soluzioni di monitoraggio continuano a fornire supporto respiratorio salvavita negli ospedali e nelle case dei pazienti.
Emergency practices
The AirSense devices are indicated for up to 15L/min. The addition of oxygen may affect device performance with increasing O2 levels. Mitigation of risk can be achieved through external monitoring.
Testing and clinical assessment by ResMed showed the ventilator performance was acceptable, but there may be some impacts when Lumis, AirCurve, AirSense and S9 devices are used with a low dispersion circuit and with an oxygen flowrate of higher than 15L/min
With increasing O2 flow levels, triggering may be less sensitive to patient efforts and trigger delay may be increased. This may lead to asynchrony for very low patient flows, but can be improved by increasing the trigger sensitivity.
Pressure delivery and device reported values may be impacted by increasing O2 flow levels, but not to an extent where patient safety is impacted or accuracy is significantly reduced.
Please refer to the following Clinical Bulletin for more information about Cautions and Warnings:
CB#010 Supplemental Oxygen and Low Dispersion Circuit with CPAP and Bi-level Devices for COVID-19
The AirSense, AirCurve, S9 Elite and S9 AutoSet devices are indicated for OSA.
With increasing O2 flow levels, triggering may be less sensitive to patient efforts and trigger delay may be increased. This may lead to asynchrony for very low patient flows, but can be improved by increasing the trigger sensitivity.
Pressure delivery and device reported values may be impacted by increasing O2 flow levels, but not to an extent where patient safety is impacted or accuracy is significantly reduced.
Please refer to the following Clinical Bulletin for more information about Cautions and Warnings:
CB#010 Supplemental Oxygen and Low Dispersion Circuit with CPAP and Bi-level Devices for COVID-19
The AirSense and AirCurve devices are indicated for OSA.
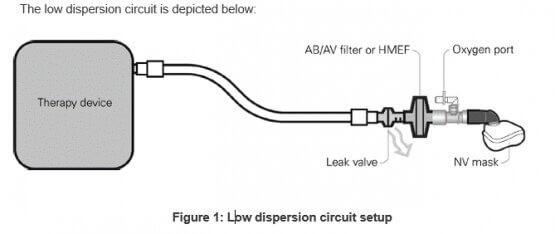
To achieve better levels of FiO2, and to minimize interferences with proper device operation, it is recommended to insert the oxygen connector directly into the patient’s mask.
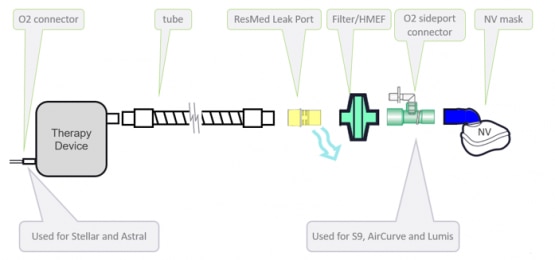
The low dispersion circuit features, in succession: a non-vented mask, an oxygen sideport connector, an antibacterial/antiviral filter or Heat Moisture Exchange Filter, a ResMed leak valve and standard tubing.
Circuit accessories (eg, AB/AV filter, oxygen port, non-vented mask) placed between the leak port and the patient contribute to increased dead-space which may lead to some rebreathing. In order to minimize this, clinicians may consider switching to bi-level mode instead of CPAP and/or increasing pressure support if patient CO2 levels increase.
Supplemental oxygen can be entrained via the dedicated oxygen inlet, located at the rear of the device. Oxygen supply should be limited to a maximum of 30 L/min. Instructions to add supplemental oxygen can be found in the Stellar clinical guide.
ResMed strongly recommends supplying oxygen through Stellar’s oxygen inlet at the rear of the device. Entraining oxygen elsewhere, i.e. into the breathing system via a side port or at the mask, has the potential to:
• Impair triggering and accuracy of therapy/monitoring and alarms (e.g., High Leak alarm, non-vented mask alarm). Therapy and alarm operation must be verified each time oxygen flow is adjusted if oxygen is not entrained through the inlet at the rear of the device.
• Promote false triggering of the blocked tube alarm leading to ventilation cessation. Therapy and alarm operation must be verified each time oxygen flow is adjusted if oxygen is not entrained through the inlet at the rear of the device.
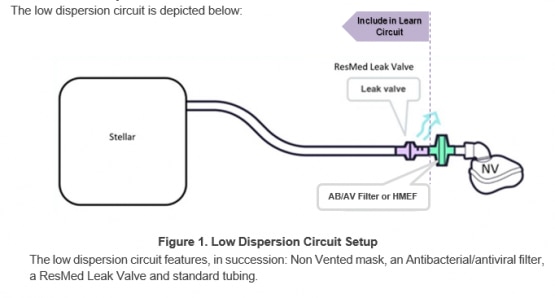
The low dispersion circuit features, in succession: a non-vented mask, an oxygen sideport connector, an antibacterial/antiviral filter or Heat Moisture Exchange Filter, a ResMed leak valve and standard tubing.
Circuit accessories (eg, AB/AV filter, oxygen port, non-vented mask) placed between the Leak Port and the patient contribute to increased dead-space which may lead to some rebreathing. In order to minimize this, clinicians may consider switching to bi-level mode instead of CPAP, and/or increasing pressure support if patient CO2 levels increase.
This low dispersion circuit configuration deviates from Stellar’s indications for use in the AB filter location, Leak Valve in non-invasive use and the use of a non-vented mask. The oxygen entrainment remains unchanged from the indications for use.
Proximal AB/AV or HMEF Filter resistance: The filter resistance or an increase in filter resistance could reduce delivered airway pressure and increase the patient’s work of breathing. Clinicians should assess the need to compensate for filter resistance if necessary by increasing set pressure levels or pressure support to overcome any additional work of breathing.
AB/AV Filters and HMEFs should be replaced according to the manufacturer’s instructions, including when impacted by moisture or secretions.
FiO2:
ResMed performed bench testing to provide users with guidance on the expected FiO2 when using the recommended circuit.
When interpreting FiO2 values, it is important to keep in mind that various factors can influence the end results, both in bench testing and in the clinical application.
Both the pressure settings and the resulting minute ventilation create variation of at least 20% FiO2. Additional variation may be caused by other confounding factors including, but not limited to: patient breath rate, inspiratory peak flows, unintentional leak, and intentional leak of the ResMed leak valve.
The low dispersion circuit is not the intended use for these devices, but emerging clinical practice in treating COVID-19 shows that it is a likely need given the ventilator shortage and the concern of infection risk to healthcare workers during the COVID-19 pandemic.
The shown circuit configuration in the clinical bulletin applies to continuous positive airway pressure (CPAP), spontaneous (S), spontaneous timed (ST), pressure assist control (PAC), and timed (T) modes in Lumis, AirCurve, AirSense and S9 platforms. These devices are identified differently throughout global regions.
The clinical bulletin provides clinicians treating COVID-19 patients with factors to consider when seeking methods to reduce the dispersion of aerosolized virus, and increase FiO2 in the healthcare setting.
The AirSense, AirCurve, S9 Elite and S9 AutoSet devices are indicated for OSA
The low dispersion circuit is not the intended use for these devices, but emerging clinical practice in treating COVID-19 shows that it is a likely need given the ventilator shortage and the concern of infection risk to healthcare workers during the COVID-19 pandemic.
Patient and device instructions from the user and clinical guide for Stellar should continue to be followed in addition to the information provided within the clinical bulletin.
The clinical bulletin provides clinicians treating COVID-19 patients with factors to consider when seeking methods to reduce the dispersion of aerosolized virus using the Stellar 100 and Stellar 150 ResMed devices.
Low dispersion circuit setups and invasive ventilation have been recommended to minimize virus aerosolization and reduce concerns for infection risk to healthcare workers during the COVID-19 pandemic. The ResMed leak valve has been recommended as part of the low dispersion circuit accessories, as well as in invasive ventilation. However, due to the high demand, this component is now in short supply.
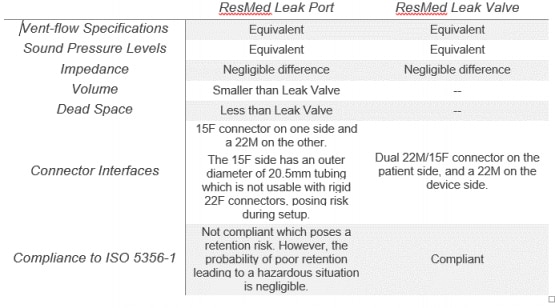
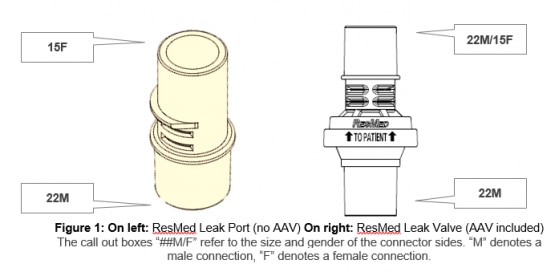
The Resmed leak valve features an integrated anti-asphysxia valve (AAV); the ResMed leak port does not.
The use of the ResMed leak port applies only to non-invasive and invasive low dispersion circuits with ResMed devices that contain alarms.
Patient and device instructions should continue to be followed in addition to the information provided within the clinical bulletin. ResMed’s user and clinical guides are not being modified for these devices. Moreover, indications for use for ResMed’s alarmed devices have not been modified.
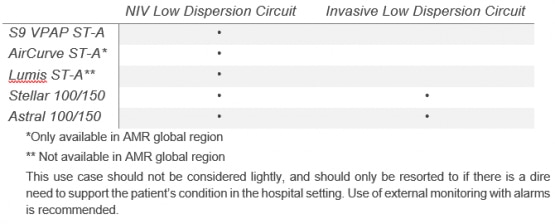
ResMed has assessed the risks of using a ResMed leak port without an anti-asphyxia valve (AAV) in place of a ResMed leak valve in ventilator breathing circuits, and has determined the clinical risk-benefit to be acceptable for the following alarmed devices and respective setups:
Please refer to the following Clinical Bulletin for more information about Cautions and Warnings:
The AirSense, AirCurve, S9 Elite and S9 AutoSet devices are indicated for OSA
The most notable difference in using the ResMed leak port in place of the ResMed leak valve is the absence of the anti-asphyxia valve (AAV), which mitigates the risk of rebreathing in the event of device failure. It is imperative to note that using the ResMed leak port for circuit configurations applies only if ResMed leak valves are not available during the COVID-19 pandemic.
The following table presents performance and dimensional feature differences between the ResMed leak port and ResMed leak valve
Please refer to the following Clinical Bulletin for more information about Cautions and Warnings:
Non-invasive low dispersion circuit setup with a ResMed leak port can be used with S9 VPAP ST-A, Lumis ST-A, AirCurve ST-A, Stellar 100/150, and Astral 100/150.
This non-invasive setup and its components can be seen in the figure below.
It is crucial that clinician ensures proper mask fit of the non-vented mask to prevent mask leak in this circuit configuration.
Please refer to the following Clinical Bulletin for more information about Cautions and Warnings:
The AirSense, AirCurve, S9 Elite and S9 AutoSet devices are indicated for OSA
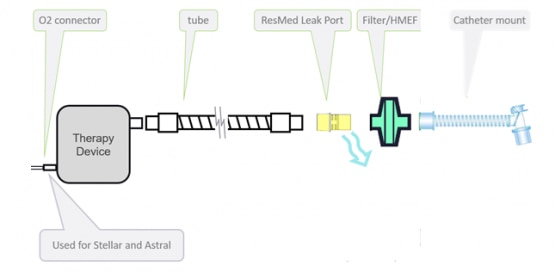
The invasive low dispersion circuit with ResMed leak port can be used only with Stellar 100/150 and Astral 100/150, using an uncuffed or deflated cuff tracheostomy tube, or endotracheal tube.
The invasive use case should not be considered lightly, and should only be resorted to if there is a dire need to support the patient’s condition.
The invasive circuit setup can be seen in the figure below.
Note that the catheter mount is not a ResMed component.
Oxygen
Astral and Stellar are able to entrain supplemental oxygen through the device via an oxygen port. These devices are designed to be compatible with supplemental oxygen up to a level of 30 L/min.
Lumis devices are compatible with supplemental oxygen of up to 4 L/min in iVAPS mode, or up to
15 L/min in all other modes.
At a fixed rate of supplemental oxygen flow, the inhaled oxygen concentration will vary depending on the ventilation mode and settings, patient breathing pattern, mask selection, and leak rate.
FiO2
Astral:
When an adult patient is using a non-vented circuit (double limb and single limb with valve, invasive and non invasive), almost 100% FiO2 can be achieved with an additional 30L/min of supplemental oxygen and more than 80% FiO2 can be achieved with an additional 15L/min of supplemental oxygen.
When an adult patient is using a vented circuit (Leak system), almost 75% FiO2 can be achieved with an additional 30L/min of supplemental oxygen, and more than 60% FiO2 can be achieved with an additional 15L/min of supplemental oxygen.
Stellar:
When adding 30L/min of supplemental oxygen, more than 90% FiO2 can be achieved if there is no leak, and almost 80% with a leak of 20L/min.
Astral and Stellar
To add supplemental oxygen, please follow the instructions below:
- Unlock the low flow oxygen inlet at the rear of the device by pushing up on the locking clip.
- Insert one end of the oxygen supply tubing into the oxygen port connector. The tubing will automatically lock into place.
- Attach the other end of the oxygen supply tubing to the oxygen supply.
- Start ventilation.
- Turn on oxygen and adjust (at the oxygen supply) to the prescribed flow rate or FiO2 level.
For Astral devices:

For Stellar devices:

To remove supplemental oxygen:
Before you remove supplemental oxygen from the device, ensure the oxygen supply has been turned off.
- Unlock the low flow oxygen inlet at the rear of the device by pushing up on the locking clip.
- Remove the oxygen port connector from the low flow oxygen inlet.
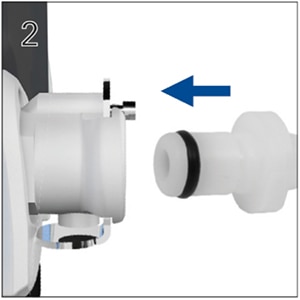
A low pressure oxygen connector (product code: 24971) is needed to connect the low flow oxygen inlet to the oxygen supply tubing. Please see below.
Lumis
To connect supplemental oxygen to Lumis devices, you can either use a ClimateLineAir Oxy tube or an oxygen connector port. For more information on how to set up the device with supplemental oxygen, refer to the user guide supplied with the accessory you choose to use.
Please note:
- Adding oxygen may affect the delivered pressure and accuracy of the displayed and reported values (e.g. leak, minute ventilation and AHI).
- Always make sure that the device is on, and airflow generated, before the oxygen supply is turned on. Always switch off the oxygen supply before turning off the device. Unused oxygen can accumulate within the device enclosure and presents a fire hazard.
Filters
For all devices (Astral, Stellar and Lumis), it is recommended that an antibacterial Heat Moisture Exchange Filter (HME) or antibacterial filter will be used to reduce the risk of contamination. While ResMed do supply these filters, other manufacturers can provide similar filters with the same antiviral/antibacterial properties.
To prevent the risk of cross-contamination, it is essential that an antibacterial filter is placed on the inspiratory port if the device is to be used on multiple patients .
Please note that the filters need to be changed frequently (for more precise information please refer to the filter user instruction) and also when the devices are used on multiple patients.
In addition, patients should follow ResMed’s user guide.
To avoid cross contamination, an antibacterial filter must be placed at the inspiratory port of Astral devices, or the air oultet of the Stellar and Lumis devices. The air tubing must be attached to the other side of the filter.
In Astral devices, antibacterial filters can be added to both the inspiratory port and the expiratory inlet. This is recommended when using nebulisers in order to protect the device.
Antibacterial HME Filter
The antibacterial HME filter must be placed between the patient end of the circuit and the patient interface.
Patient interfaces include any components placed after the single circuit’s expiratory valve or exhalation port, or a double limb circuit’s Y piece. Where there is a ResMed leak valve, connect the antibactierial HME filter to the patient side of this.
HMEs are passive humidification systems which retain heat and moisture from the patient’s exhaled gases via an internal membrane. An HME should not be used with active humidification.
In addition, patients should follow ResMed’s user guide.
Astral: the machine filter filters particles of 10 micrometers and above. It is a dust filter and has to be changed between patients.
Stellar: The machine filter in the Stellar device has a bacterial filtration efficiency of 99.540% on area weight 100g/m². As viruses are typically smaller than bacteria (<0.1 micrometers), the filter is not guaranteed to remove them from the entrained air. The filter has to be changed between patients.
Lumis: The standard air filter in Lumis devices is made of non-woven polyester. The average efficiency is >75% for >7 micrometers dust. The hypoallergenic filter is made of acrylic and polypropylene fibres, in a polypropylene carrier. Its efficiency is >98% for >7-8 micrometers dust; >80% for >0.5 micrometers dust. Both types of filter have to be changed between patients.
Please note: If using an antibacterial filter, it must be checked for signs of moisture or other contaminants, particularly during humidification. Failure to do so could result in increased breathing system resistance.
Further instruction for using Stellar with an external humidifier:
Place the antibacterial filter at the air outlet, connect the external humidifier to the other side of the AB filter through the air tubing. Connect the other air tubing from the humidifier to the ResMed leak valve.
Do not use an antibacterial filter with the internal humidifier H4i.
In addition, patients should follow ResMed’s user guide.
HEPA filters remove at least 99.95% of particles with a diameter equal to 0.3 micrometers from the air which passes through.
Antibacterial and antibacterial HME filters filter particles >0.1 micrometers, meaning that they are more effecitive at filtering particles and microorganisms than HEPA filters.
In addition, patients should follow ResMed’s user guide and clinical guide.
To protect the Astral air inlet in a hospital setting, staff should ensure the air filter is not blocked by clothes or other objects, inspect the air inlet filter regularly and replace if necessary.
In addition, patients should follow ResMed’s user guide.
Disinfection
Information about cleaning and disinfecting the external device surfaces can be found in the clinical guide. However, as COVID-19 is a novel virus, specific decontamination methods have not yet been validated.
In addition, patients should follow ResMed’s cleaning procedures, as outlined in the user guide.
The Cleaning and Disinfection section of the ApneaLink Air Clinical Guide describes the validated materials and method for processing devices between patients. It is not necessary to clean the interior of the ApneaLink Air device. Based on the behaviours of this virus, and its ability to spread, ResMed has assessed the risk of cross-infection for homecare devices and determined it to be improbable. This is based on:
- the difficulty of contamination reaching the device interior, and
- the ability to the virus to remain viable on a surface in that environment, and
- Current survival time on surfaces is approx. three days1
- Current survival time as an aersol is approx. three hours1
- the ability of the virus to be mobilised and transmitted to a second patient. As described by the WHO, the key transmission risk for COVID-19 is via person to person transmission in close contact.
[1] Aerosol and surface stability of HCoV-19 (SAR-CoV-2) compared to SARS-CoV-1. Van Doremalen et al 2020
ResMed has not tested or validated the use of in-line filters for the ApneaLink Air, and therefore cannot recommend the use of these filters with the product.
Information & communication
Detailed instructions are available on the product pages, and support pages, of our corporate website. You will find a range of official guides for ResMed products, including Set-Up for our ventilation machines. Clinical guides will be on demand.
For further information, try our free education platform, ResMed Academy Online. Here, you can access a rich bank of educational resources, learning activities and expertise, designed to help improve patient outcomes.
If you do not already have an account, you can sign-up to ResMed Academy Online at no cost; the registration process is simple and should take no longer than a couple of minutes.
ResMed will soon be publishing more educational resources on the webinar.resmed.eu site, so please check back for further information soon.
For any further questions, please contact your local customer or sales representative.
We recognise that many of you have concerns regarding orders, supply and shipment in light of the coronavirus pandemic, and we are committed to providing regularly updated information to our customers across the globe. To help ensure our communications reach the community as soon as possible, we have created a dedicated COVID-19 FAQ page, which will be updated as and when new information becomes available to us.
For any further questions, please contact your local customer or sales representative.
How to prepare for COVID-19: useful information from health organizations
As the coronavirus (COVID-19) outbreak unfolds across the globe, we endeavour to support patients, customers and healthcare professionals as they navigate this challenging time. ResMed is committed to providing all possible guidance and education for our global customer base.
To aid access to relevant and verifiable information about COVID-19, we are signposting to a range of official websites. These reliable sources publish data, research findings and public health advice regularly and, wherever possible, are country specific.
We appreciate that due to the swift progression of the virus, best-practice is changing rapidly. By providing these links, we hope to guide our customers to real-time updates to assist them in their response.
The World Health Organisation (WHO) educates and informs citizens worldwide on a range of health matters. During a health crisis, the WHO provides invaluable research and situation reports to track events as they evolve.
European health agencies differ in their advice surrounding containment, treatment and public health restrictions with regard to COVID-19. To help you find the most relevant information, please click on a country-specific link below to find out what your healthcare authority recommends.
- Europe-wide
European Respiratory Society (ERS)
European Society of Intensive Care Medicine
European Society of Anaestesiology (ESA)
- UNITED KINDGOM
British Thoracic Society (BTS)
The Faculty of Intensive Care Medicine (FICM) – for critical care guidance
For healthcare professionals, we are working diligently to source the most pertinent research on COVID-19. The list below will be updated to reflect emerging insights from the medical field, as part of our commitment to support those working on frontline or ongoing response efforts.
The Society of Critical Care Medicine (SCCM) is US-based body which provides resources and updates for those working in the critical care community during times of disaster and emergency.
https://www.sccm.org/disaster?utm_source=COVID19_SSC
To access guidance for clinicians from the SCCM:
“Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19)” https://www.sccm.org/getattachment/Disaster/SSC-COVID19-Critical-Care-Guidelines.pdf?lang=en-US
For information from the SCCM on possible treatment pathways for Covid-19 patients with hypoxia:
https://www.sccm.org/getattachment/Disaster/SCCM-COVID-19-Infographics1.pdf?lang=en-US
For information from the SCCM on various treatment pathway guidelines for Covid-19 patients:
https://www.sccm.org/getattachment/Disaster/SCCM-COVID-19-Infographics2.pdf?lang=en-US